TL; DR Quite well, if you use an insulated container with a lid. Just like their FAQ says. But a mug with a lid is still an improvement, as long as you don’t linger over it for too long.
I’ve been meaning to try Coffee Joulies ever since they came out a couple of years ago, and I finally got around to it. Problem is, it’s hard to tell how much difference they make. Sure, I know what their site says. But that might just be marketing. I want the data. So I made it.
Disclaimer: IANAS (I Am Not A Scientist)
Methodology
I decided to measure the rate of temperature fluctuation in three scenarios. Each scenario was run with Joulies and without:
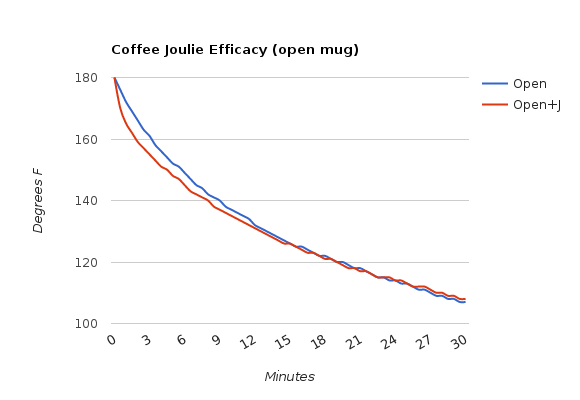
- In a regular, open mug
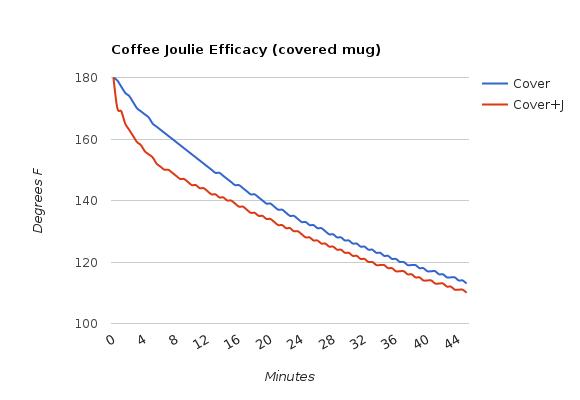
- In a regular mug, covered with a lid
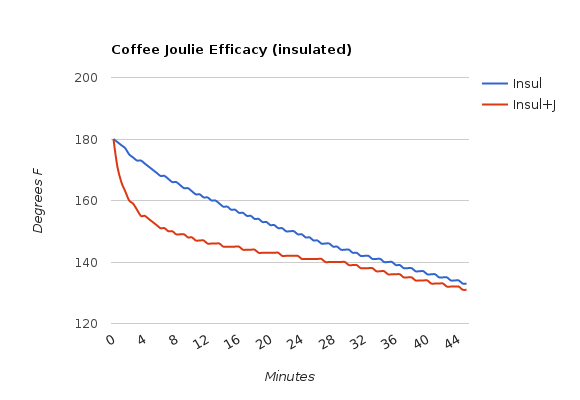
- In an insulated mug, lid included
I used 8oz of water and 2 Joulies (1 Joulie per 4oz is recommended). I used a standard kitchen digital thermometer and took readings every 30 seconds. I measured starting at 180 degrees F. I began doing multiple runs and averaging the results, but the numbers were so similar I decided it wasn’t worth the effort. This is 1st World Science after all.
Results
The results seem fairly clear, though I’m sure the specific numbers will vary with different types of containers and different starting temperatures. In the legend, “+J” indicates that Joulies were used.
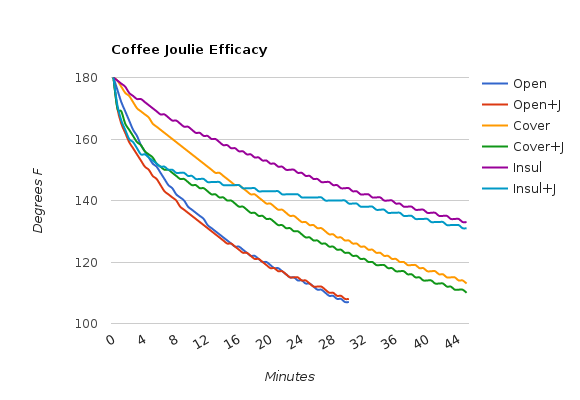
Individual Results
Conclusion
Using Joulies in an insulated mug with a lid is clearly the way to go, just as their FAQ suggests. I suspect a higher starting temp and a better quality of insulated mug will yield even more dramatic results. Joulies in a regular mug with a lid work well, just not for nearly as long. And Joulies in a mug with no lid? It will cool down to drinking temp a few minutes faster, but it won’t hold heat any longer than a mug without Joulies.
Raw Data
And just ‘cause we’re having fun, here’s my raw data.